gcsescience.com 44 gcsescience.com
What is Metallic Bonding?
What is the Structure of a Metal?
Metals form a giant
structure
(like ionic compounds and giant molecules).
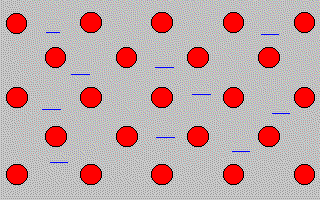
In the picture below the red balls represent metal
ions.
The blue
lines represent
delocalised electrons
in the
outer shell of the
metal ions.
Delocalised means that the
electrons are
not attached to one particular
ion.

The delocalised electrons
between the positive metal ions
hold the structure together
by strong electrostatic forces.
The delocalised electrons in the structure
of
a metal are sometimes called a "sea of
electrons".
The structure of a metal
can also be shown as

In the above picture the dotted lines
represent the delocalised
electrons.
Delocalised electrons are also called
free electrons
because they can
move very
easily through the metal structure.
It is these free electrons which give
metals their properties.
![]() Links
Revision Quizzes
Revision Questions
Links
Revision Quizzes
Revision Questions
![]()
gcsescience.com The Periodic Table Index Atomic Structure Quiz gcsescience.com
Home GCSE Chemistry GCSE Physics
Copyright © 2015 gcsescience.com. All Rights Reserved.