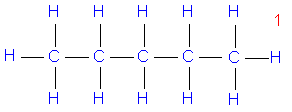
the straight chain normal structure for pentane.
gcsescience.com 25 gcsescience.com
What are the Isomers of Pentane?
Pentane (C5H12) has three structural isomers.
Isomer 1 is
n-pentane,
the straight chain normal structure for pentane.

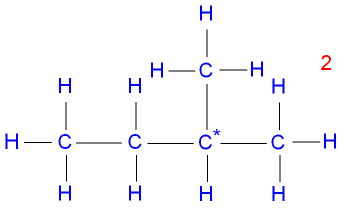
Isomer 2 is
2-methylbutane, a branched chain
with
a carbon* atom joined onto
three other carbon atoms.
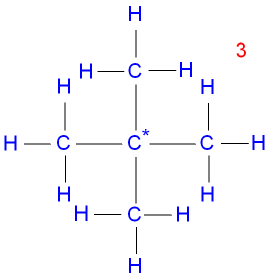
Isomer 3 is
2,2-dimethylpropane, a
branched
chain with the
central carbon* atom joined onto
four other carbon atoms.
Note that for all the isomers,
each
carbon atom has four bonds (valency
4),
and each hydrogen atom has one bond
(valency 1).
Valency is the combining power of an atom.
You cannot change the number of
bonds
that each
atom has, see the isomers
of pentane.
![]() Links Hydrocarbons Revision Questions
Links Hydrocarbons Revision Questions ![]()
gcsescience.com The Periodic Table Index Hydrocarbons Quiz gcsescience.com
Home GCSE Chemistry GCSE Physics
Copyright © 2015 gcsescience.com. All Rights Reserved.