gcsescience.com 11 gcsescience.com
Elements, Compounds and Mixtures
Changing from a Solid to a Liquid to a Gas by Heating.
Any substance may exist as a solid, liquid or gas.
Heating and Cooling a Solid.
If a solid is
heated enough, it
will melt to become a liquid.
The temperature at which
it melts is called its melting point.
If the liquid is then cooled, it will freeze to become a solid again.
The temperature at which it
freezes is called its freezing point.
The melting point and the freezing point
is the same for the same
substance.
Sometimes a heated solid will
turn into a gas
without first becoming a
liquid. This is called sublimation.
Examples of solids that sublime are iodine and carbon dioxide.
Heating and Cooling a Liquid.
If a liquid is
heated enough, it
will boil to become a gas.
The temperature at which it
boils is called its boiling point.
If the gas is then cooled, it will condense to become
a liquid again.
A gas will condense at its boiling
point.
A liquid can also become a gas by evaporation.
This happens at a temperature below its boiling
point.
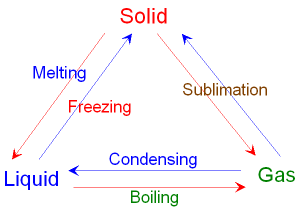
Interconverting between a Solid, Liquid and Gas.
The figure below shows
interconverting
from
one state
to another by heating or cooling.
Red arrows involve heating, blue arrows
involve cooling.

The state of a substance
(whether it is solid, liquid or gas)
depends on its temperature, the
RFM of the
particles
and the forces of attraction
between the particles.
![]() Links
Solid
Liquid
Gas
Revision Questions
Links
Solid
Liquid
Gas
Revision Questions
![]()
gcsescience.com The Periodic Table Index Chemistry Quiz gcsescience.com
Home GCSE Chemistry GCSE Physics
Copyright © 2015 gcsescience.com. All Rights Reserved.