gcsescience.com
19
gcsescience.com
Atomic Structure
How to Balance
Chemical Equations - Example
1.
When we are balancing
chemical equations we first
need to know
what the symbols and numbers
mean.
Click
here if you are not sure.
Example 1 is the reaction between potassium and chlorine.
(1)
K + Cl2
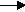 KCl
KCl
On the left side of the arrow, there is 1K and 2Cl.
The two chlorines in Cl2
cannot be changed,
since Cl2 is the formula of chlorine.
On the right side of the arrow, there is 1K and 1Cl.
The reaction is not
balanced
because atoms are not
gained or lost
in a chemical reaction.
The right side needs an extra
chlorine.
A big
number in front of the
symbol
changes the number of
elements in the formula
that follows it.
2K means two potassium ions. 2Cl2 means
four chlorine atoms.
2KCl
means two potassium ions with
two chlorine ions.
To get an extra chlorine on the
right side, we can put a 2 before KCl.
The equation becomes
(2) K + Cl2
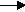 2KCl
2KCl
Now the equation is balanced for chlorine, 2 on each
side.
But the equation has now become unbalanced for potassium.
There is 1 on the left and 2 on the right.
To get an extra potassium on the left, we can put a 2 before
K.
(3) 2K + Cl2
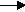 2KCl
2KCl
The equation is now balanced.
On the left there are 2 potassiums and
2 chlorines.
On the right there are 2 potassiums
and 2 chlorines.
Showing the three steps together, we have
(1)
K + Cl2
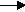 KCl unbalanced for chlorine.
KCl unbalanced for chlorine.
(2)
K + Cl2
 2KCl unbalanced for potassium.
2KCl unbalanced for potassium.
(3) 2K + Cl2
 2KCl
Balanced!
2KCl
Balanced!
See example 2. The
reaction of lithium
with oxygen.
 Links
Revision Quizzes
Revision Questions
Links
Revision Quizzes
Revision Questions
gcsescience.com
The Periodic Table
Index
Balancing Equations Quiz
gcsescience.com
Home
GCSE Chemistry
GCSE Physics
Copyright © 2015 gcsescience.com. All Rights Reserved.